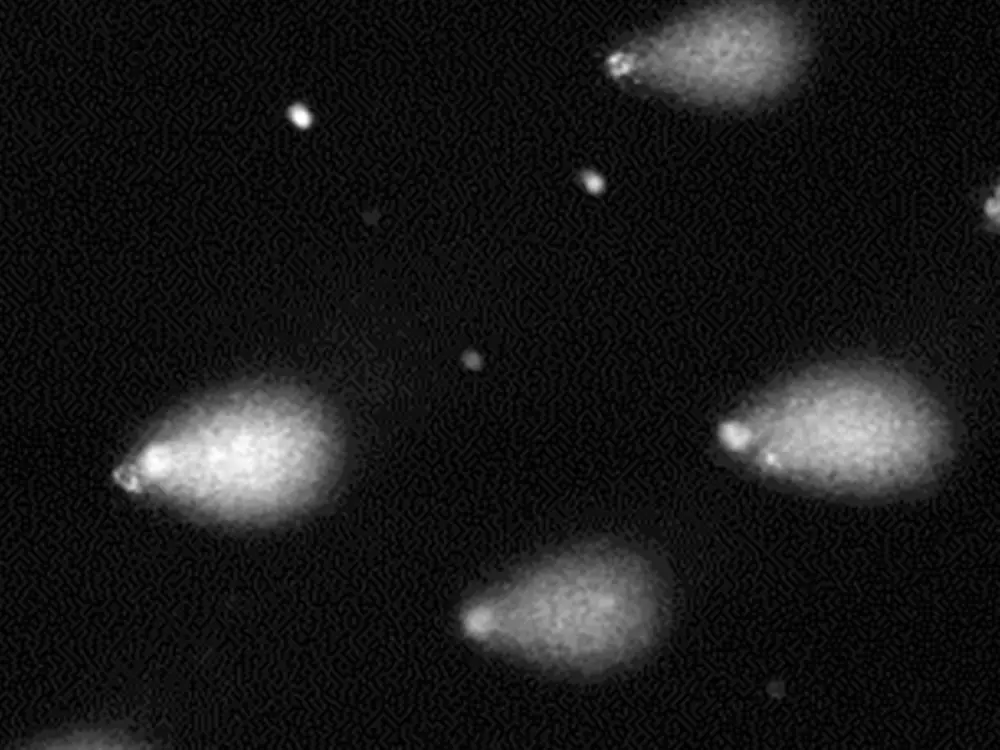
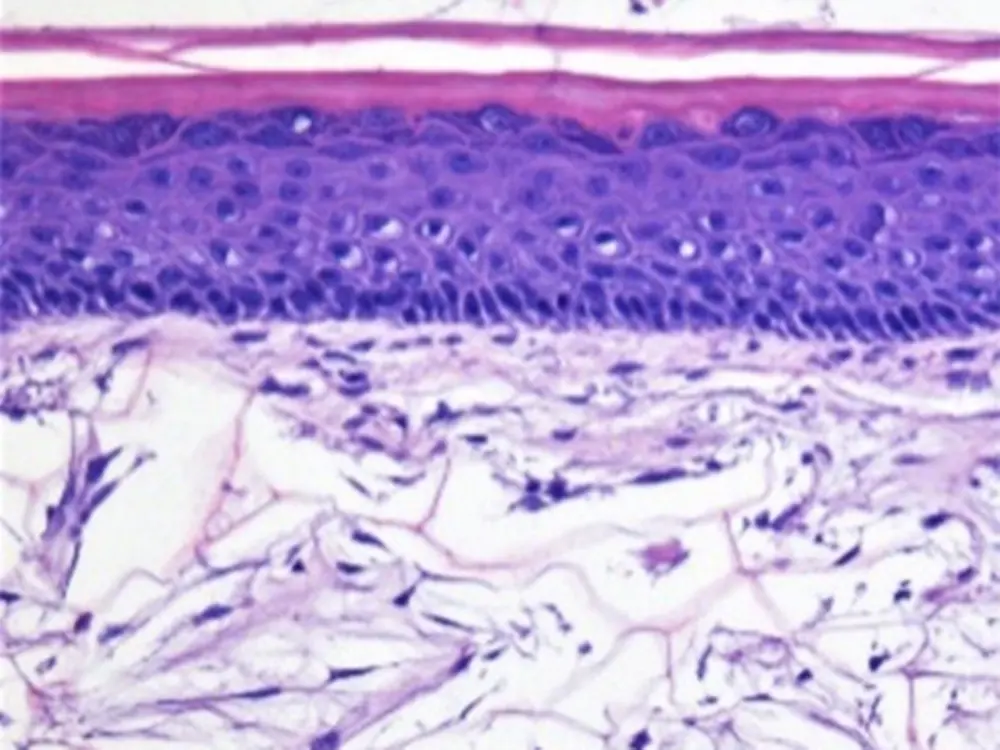
Charles River Laboratories in Senneville, Canada, have successfully established the 3D Skin Comet assay (in a GLP and non GLP format) to assess the genotoxic potential of chemicals. The 3D Skin Comet assay addresses two underrepresented aspects in classical in vitro genotoxicity assays, a species and organ-specific xenobiotic metabolism, and the relevant route of exposure. With this test system, Charles River adds another tool to its already existing portfolio of in vitro test methods for the toxicological risk assessment of chemicals. For over 30 years, Charles River has been helping clients develop custom testing strategies and conduct appropriate assays to meet the unique requirements of their chemicals and drugs.
Nov 10, 2021
Charles River Laboratories, Senneville, Canada, Successfully Establishes 3D Skin Comet Assay
The 3D Skin Comet Assay is intended to be used as a follow-up method after positive results from other genotoxicity tests. For the first time, it addresses the dermal route of exposure, which is especially important in terms of cosmetic ingredients.
The innovative in vitro test is based on the Phenion® Full-Thickness Skin Model, comprising an epidermis and a dermis, both constructed from metabolically competent primary human skin cells. After successfully completed validation studies, the 3D Skin Comet assay was included in the “OECD Work plan for the Test Guidelines Program (TGP)” in 2020.
The implementation of the 3D Skin Comet assay at Charles River was supported by an expert team of the Henkel GmbH & Co. KGaA in Germany, the producer of the Phenion® Full-Thickness Skin Model.
For more information about the 3D Skin Comet assay, please visit Genetic Toxicology Studies | Charles River (criver.com)