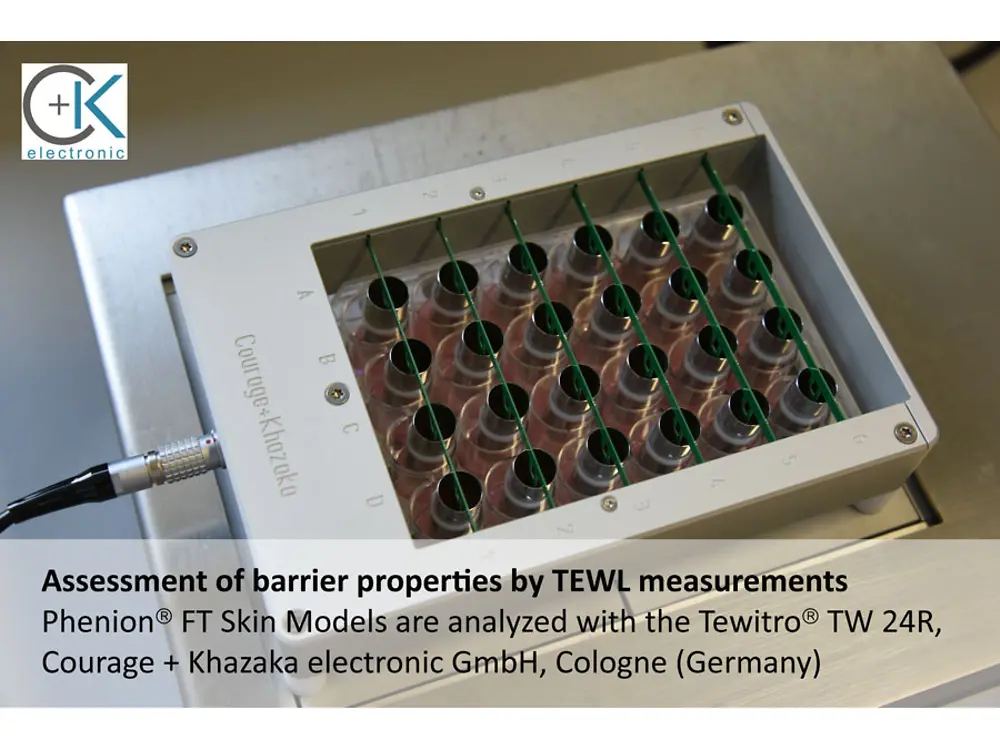
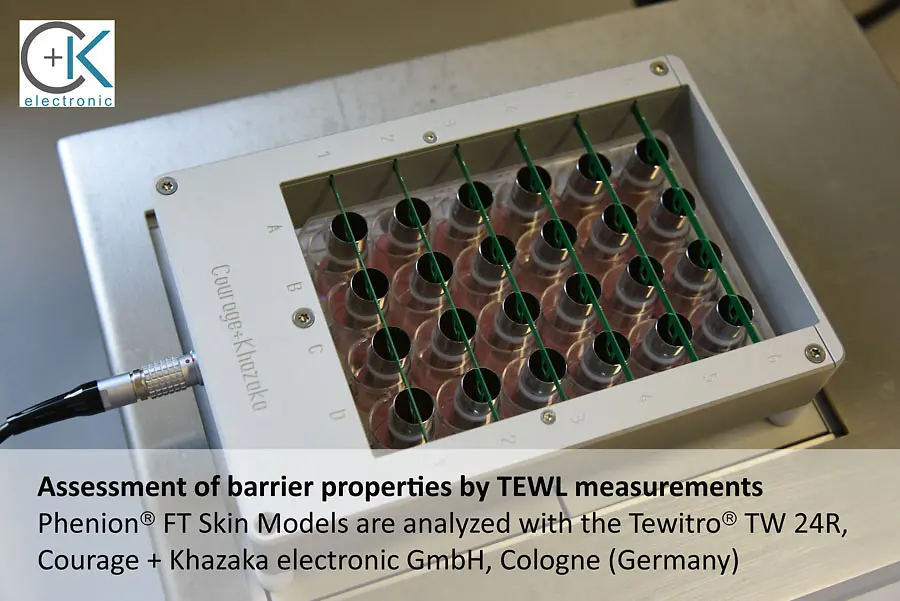
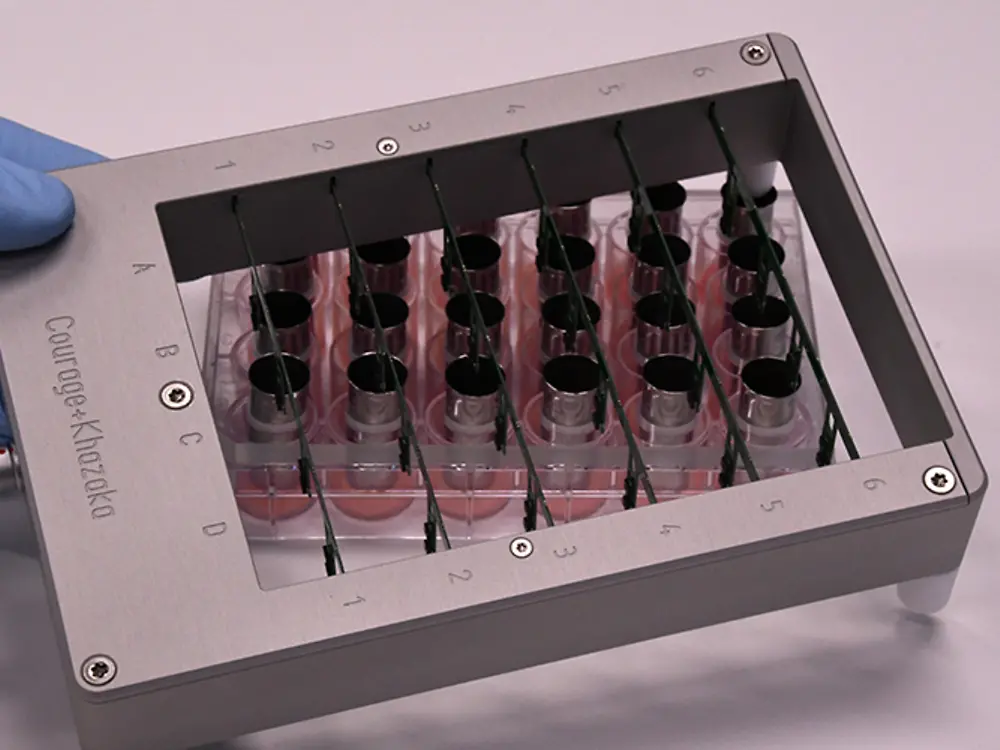
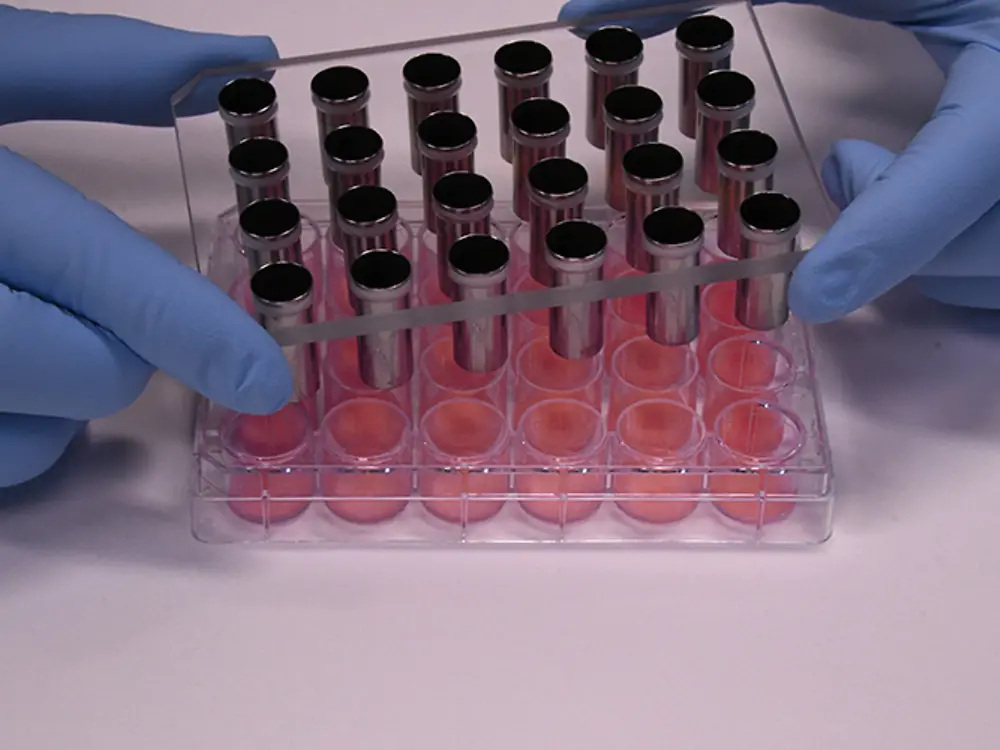
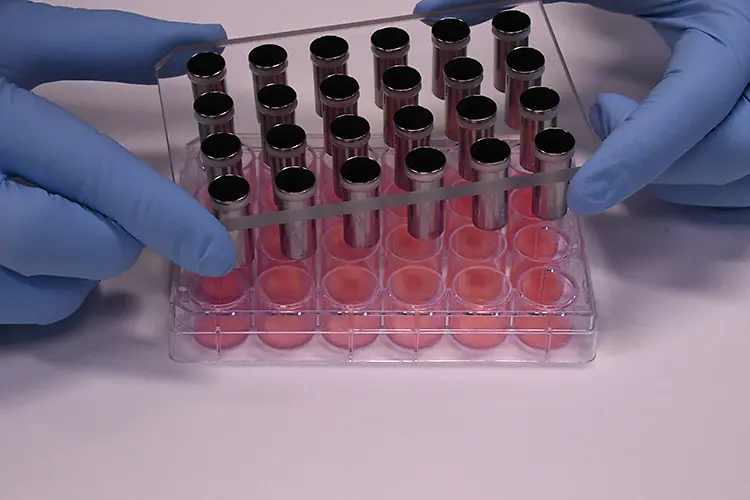
The Tewitro® TW 24R device, developed by Courage + Khazaka electronic GmbH, Cologne, has proven its value to reliably measure the transepithelial water loss (TEWL) in 3-dimensional human Phenion® Full-Thickness Skin Models. The TEWL is a meaningful parameter to assess the physiological status of the dermal barrier, which, if intact, protects our skin against environmental impacts and prevents undesired water loss.
Jan 13, 2020 Düsseldorf / Germany
NEW: Non-invasive technology for the evaluation of skin barrier properties in Phenion® FT Skin Models
With the innovative sensor panel, up to 24 skin models can be examined in parallel. This new set-up uses the advanced and world- wide established Tewameter® technology commonly used in dermatological research, and it can be conveniently conducted in a standard 24- well plate.
Each TEWL unit comprises two sensors which continuously detect temperature and humidity in the wells. The differences between the values detected with both the upper and lower sensors allow the calculation of the TEWL values for all tissues in parallel. This approved principle for TEWL assessment is used in numerous in vivo studies to describe the status of the human skin barrier function.
We demonstrated that with progressing epidermal differentiation of the Phenion® FT Skin model the TEWL decreases. Once the cornified layers have reached a certain thickness, coinciding with a robust barrier, the skin models display a steady level of water evaporation.
As a proof of concept, the TEWL was determined for full-thickness skin models which had been topically treated with SDS solution (0.5%, 1%, 2%). The observed TEWL values during a period of 4.5 hours after treatment mirrored the compromised skin barrier of the tissue models due to the properties of the surfactant.
With this study we have successfully proven that the C+K Tewitro® TW 24R is well-suited to non-invasively monitor TEWL of the Phenion® FT Skin Model. These results open up new opportunities to monitor the dermal barrier physiology under different experimental conditions and treatments in vitro.
For further information about the TEWL device C+K Tewitro® TW 24R, visit the website of Courage + Khazaka electronic GmbH (www.courage-khazaka.de).